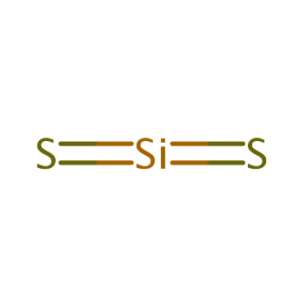Let’s Shape the Molecules
Why does the molecule shape important?
The molecule is very important in terms of size and shape because each characterizes different function in cells.
VSEPR Model: Use to predict the shapes and angles of the molecule using their “pair electrons that surround the central molecules”
VSEPR for Lewis Structure: Valance electron, Skeleton, Electron, Pairs of bond, Review formal charge
Example: H2O
Valance electron: H2 = 1 x 2 = 2, O = 6, Sum of both valance electron is 8
Skeleton: ‒ = 2 electrons or we call it pair bond sharing between the molecule, H‒O‒H
Electron: Check it whether each molecule satisfy with their electron, Ex: H has 2 (share with O), which it is satisfying because it has the same outer shell like Helium
Pairs of bond: Check the structure whether it has more than one pair bond to share
Review Formal charge: To check all of the molecules, whether they satisfy their own needs or forget to create other pairs of bond
VSEPR for: Valance-Shell Electron-Pair Repulsion
Two bonded pairs:
Linear: only two bonded pair, 180o, non-polar
Bend: 2 bonded and 1 lone pair/s, <120o, polar
Trigonal planar: if only 3 bonded pairs, 120o, non-polar
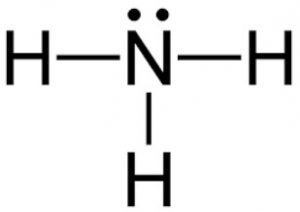
Trigonal pyramidal- 3 bonded but also a lone pair, <109.5o, Polar
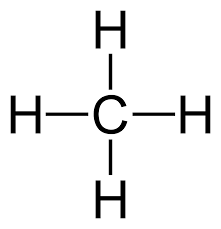
Tetrahedral: If only 4 bonded pairs, 109.5o, non-polar
See-saw: If 4 bonded and 1 lone pair, <900 and <1200, non-polar
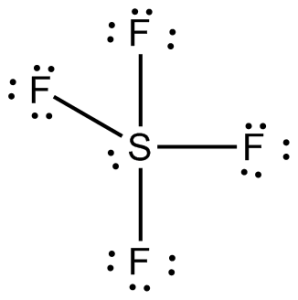
Trigonal bipyramidal: If five bonded pairs, 900 and 1200, non-polar
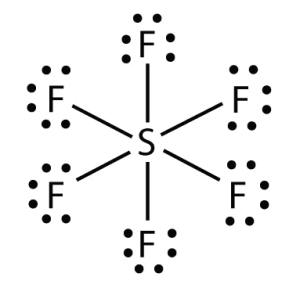
Octahedral: If six bonded pairs, 900, non-polar
Example: SiS2
- Lewis Structure
- Check the lone pair/s electron
- Identify the shape
- Add the angle
- Polar/non-polar
- There are no lone pairs
- Linear
- 1800
- Non-polar
Resources: https://www.youtube.com/watch?v=xNYiB_2u8J4